BioticsAI, a pioneering company in medical technology, has officially announced that its sophisticated artificial intelligence software designed to enhance fetal ultrasound imaging has received clearance from the U.S. Food and Drug Administration (FDA). This pivotal regulatory approval marks a significant milestone for the firm, which gained recognition as the winner of TechCrunch Disrupt’s prestigious Battlefield competition in 2023, positioning its AI-powered solution to revolutionize prenatal care by improving the detection of fetal abnormalities. The technology promises to bolster diagnostic accuracy, streamline clinical workflows, and ultimately contribute to better health outcomes for expectant mothers and their babies.
The Genesis of Innovation: A Personal Mission
The vision for BioticsAI stemmed from a deeply personal background, rooted in the healthcare experiences of its founder and CEO, Robhy Bustami. Growing up immersed in a family of obstetricians, including his mother, aunt, and uncle, Bustami spent countless hours within hospital environments. These formative years involved frequent travels alongside his mother as she provided vital maternal care across various communities in the United States. This intimate exposure to the challenges and nuances of prenatal healthcare instilled in him a profound understanding of the critical need for advancements in the field.
His academic journey at UC Irvine, where he delved into computer science and honed his coding skills, provided the technical foundation necessary to address these observed healthcare gaps. The convergence of his medical upbringing and technological expertise culminated in 2021 when Bustami co-founded BioticsAI. He brought together a talented team, including Salman Khan, Chaskin Saroff, and Dr. Hisham Elgammal, to transform his innovative concept into a tangible solution. Their collective goal was to leverage cutting-edge AI to overcome long-standing limitations in prenatal diagnostics, aiming to make a tangible difference in the lives of countless families.
Addressing a Critical Healthcare Challenge
The United States currently faces a sobering reality regarding maternal and infant health, grappling with some of the highest rates of maternal mortality among high-income nations. This crisis is particularly acute within specific demographics, with Black women experiencing disproportionately higher rates of maternal deaths. These statistics underscore a systemic challenge within the healthcare infrastructure, where access to quality care, diagnostic accuracy, and timely intervention remain critical areas for improvement.
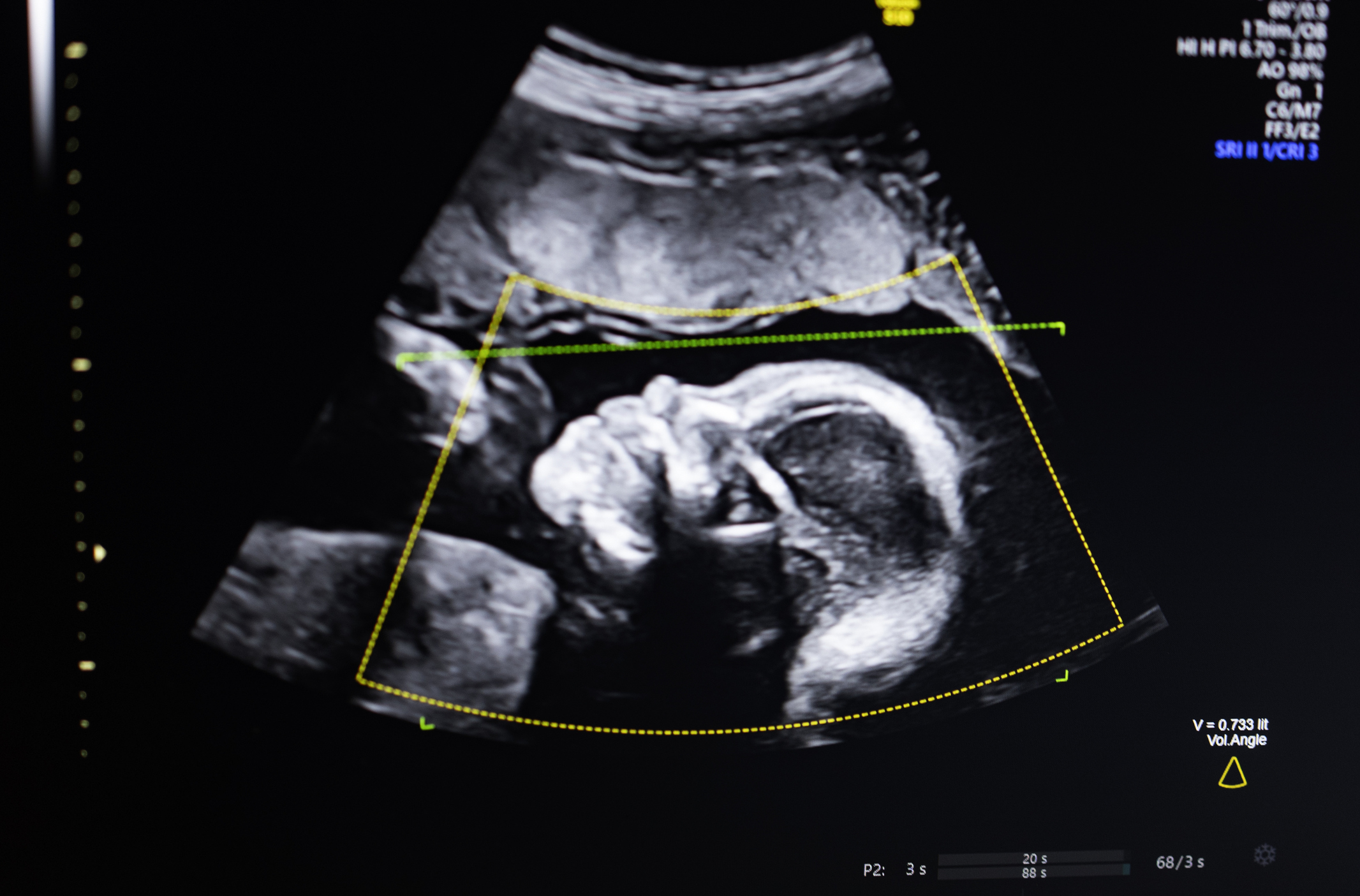
Prenatal ultrasound has long been hailed as the cornerstone of pregnancy monitoring, providing invaluable insights into fetal development and maternal health. However, the efficacy of traditional ultrasound relies heavily on the skill and experience of the operator, as well as the inherent limitations of the imaging technology itself. Low-quality images, often a result of various factors including patient body habitus, fetal position, and equipment variations, can lead to ambiguous findings or, more critically, misdiagnoses. Such inaccuracies can have profound consequences, delaying necessary interventions or causing undue anxiety for families. The manual, often subjective, nature of interpreting these complex images introduces variability, making it challenging to ensure consistent, high-quality assessments across all clinical settings. This variability contributes directly to disparities in care, particularly affecting underserved populations where access to highly specialized sonographers might be limited. BioticsAI’s technology directly confronts these challenges, seeking to standardize and elevate the quality of prenatal ultrasound examinations nationwide.
The Technology Behind the Breakthrough
At the core of BioticsAI’s innovation is a sophisticated computer vision AI system. This technology is designed to intelligently analyze ultrasound images, going beyond what the human eye might discern alone. As Bustami explained, the AI supports several critical functions: it performs quality assessment of the ultrasound images, verifies the anatomical completeness of the fetal scan, generates automated reports, and seamlessly integrates these capabilities into existing clinical workflows. This multifaceted approach aims to enhance every stage of the diagnostic process.
The development of this AI model involved rigorous training on an expansive and diverse dataset comprising hundreds of thousands of ultrasound images. This extensive training was crucial for the AI to learn to identify subtle patterns and anomalies indicative of fetal abnormalities. Bustami highlighted that the true challenge wasn’t merely building the AI models, but ensuring their reliable performance in real-world clinical environments, especially for patient demographics at the highest risk for adverse outcomes. This emphasis on real-world robustness is paramount in medical AI, where performance must be consistent across a wide spectrum of patient variability, not just in idealized, controlled scenarios. The company’s commitment to demonstrating consistent performance across various patient subgroups directly addresses concerns about algorithmic bias and ensures equitable application of the technology. By providing an objective, data-driven layer of analysis, the AI aims to reduce operator-dependent variability, making high-quality diagnostic insights more accessible and consistent, regardless of the clinical setting or the specific sonographer performing the scan. This level of standardization holds immense potential for reducing misdiagnoses and improving early detection of critical conditions.
Navigating the Regulatory Landscape
Securing FDA clearance is a formidable undertaking for any medical technology company, particularly for AI-driven solutions that introduce new paradigms in diagnostics. The process is characterized by stringent requirements for safety, efficacy, and clinical validation. For BioticsAI, this journey spanned just under three years, a testament to the complexity and rigor involved. During this period, the company underwent extensive testing and validation procedures to demonstrate the reliability and accuracy of its product across diverse clinical scenarios.
Bustami reflected on this demanding experience, emphasizing a key learning: the critical importance of tightly aligning engineering, product development, clinical validation, and regulatory strategies from the very outset. He noted that by designing the product, its clinical validation protocols, and the regulatory pathway in an integrated fashion, rather than sequentially, his team was able to navigate the process with remarkable efficiency. This concurrent approach facilitated proactive problem-solving, ensured that the technology met regulatory standards at every development stage, and streamlined the submission process. The FDA’s rigorous evaluation, which often includes examining the algorithms for potential biases and ensuring generalizability across different populations, underscores the importance of BioticsAI’s focus on consistent performance across patient subgroups. This integrated strategy not only accelerated the clearance timeline but also solidified the foundation for a robust and compliant medical device ready for widespread clinical adoption.
Market Implications and Healthcare Transformation
The FDA clearance for BioticsAI’s AI-powered fetal ultrasound product carries substantial implications for the prenatal care market and the broader healthcare landscape. This approval signals a shift towards more technologically advanced and data-driven diagnostic methods, promising to enhance both the accuracy and efficiency of prenatal examinations. For sonographers and obstetricians, the technology offers a powerful tool to augment their expertise, providing an additional layer of analytical support that can highlight potential abnormalities, ensure comprehensive scans, and reduce the cognitive load associated with manual image interpretation. This could lead to a significant reduction in scan times and an increase in throughput, allowing healthcare providers to serve more patients without compromising quality.
Beyond efficiency, the social impact of such technology cannot be overstated. By standardizing the quality assessment of ultrasounds and automating parts of the reporting process, BioticsAI’s solution has the potential to mitigate disparities in care, particularly in underserved regions or clinics with fewer highly specialized personnel. This democratization of high-quality diagnostic capabilities can empower healthcare providers in remote or resource-limited settings to offer a standard of care previously only available in major urban centers. Earlier and more accurate detection of fetal conditions can lead to timely interventions, potentially preventing severe complications, improving long-term health outcomes for infants, and reducing the emotional and financial burden on families. Economically, the prevention of complications through early diagnosis can translate into significant cost savings for healthcare systems by reducing the need for more intensive and expensive treatments later in a child’s life. This innovative approach aligns perfectly with the burgeoning trend of precision medicine, where individualized, data-driven insights are leveraged to deliver tailored and effective care.
The Broader AI in Healthcare Movement
BioticsAI’s success is emblematic of a broader, transformative trend: the increasing integration of artificial intelligence across various domains of healthcare. AI is rapidly moving from theoretical concept to practical application, revolutionizing fields from radiology and pathology to drug discovery and personalized medicine. In diagnostics, AI algorithms are demonstrating capabilities in identifying patterns in medical images, genetic data, and patient records that can surpass human capabilities in speed and sometimes even accuracy.
However, the journey of AI in healthcare is not without its challenges. Issues surrounding data privacy and security, the ethical implications of algorithmic decision-making, ensuring physician acceptance, and developing transparent, interpretable AI models remain critical areas of focus. BioticsAI’s achievement underscores the potential for AI to integrate successfully into highly sensitive and critical areas like prenatal care, provided that rigorous validation, regulatory compliance, and a deep understanding of clinical needs are prioritized. The company’s focus on ensuring consistent performance across diverse patient subgroups also addresses one of the most significant ethical considerations in medical AI: the potential for algorithms to perpetuate or exacerbate existing health disparities if not carefully developed and tested. As AI continues to mature, its role is expected to expand, offering solutions to long-standing problems and pushing the boundaries of what is possible in patient care, diagnostics, and treatment.
Future Horizons for BioticsAI
With the crucial FDA clearance secured, BioticsAI is now poised for a significant phase of expansion and innovation. The immediate strategic focus for the company is to scale its technology across various health systems nationwide. This involves establishing partnerships with hospitals, clinics, and maternal care networks to integrate their AI software into existing infrastructure, thereby extending its reach and impact. The successful deployment across diverse healthcare settings will be key to realizing the full potential of the technology in improving prenatal outcomes.
Looking ahead, Bustami also articulated plans to further enrich the product’s capabilities. The company intends to add more features specifically tailored for fetal medicine and reproductive health. This could include advanced analytical tools for more complex conditions, predictive analytics for potential risks, or enhanced reporting functionalities that provide even deeper insights for clinicians. As Bustami stated, "We’re positioned to scale both distribution and clinical impact while continuing to deepen the power of our technology." This forward-looking perspective suggests a continuous commitment to research and development, ensuring that BioticsAI remains at the forefront of AI innovation in maternal and fetal health. Beyond the domestic market, the success in the U.S. could also pave the way for international expansion, addressing similar prenatal care challenges in other regions of the world.
A New Era for Prenatal Care
BioticsAI’s FDA clearance represents more than just a regulatory triumph; it signifies a powerful step forward in the quest to enhance prenatal care and address critical health disparities. By leveraging advanced AI to improve the quality, accuracy, and accessibility of fetal ultrasound diagnostics, the company is set to play a pivotal role in transforming how pregnancies are monitored and managed. This innovation promises to empower healthcare providers with superior tools, ultimately leading to earlier detection of abnormalities, more informed clinical decisions, and significantly better health trajectories for mothers and their newborns. As artificial intelligence continues to mature and integrate into healthcare, BioticsAI stands as a beacon of its potential to foster a future where high-quality, equitable prenatal care is a reality for all.